Tudástár
Microneedles are a promising and minimally invasive transdermal delivery technique effective in promoting peptide permeation through the skin.
The needles have a size ranging from 100 to 1500 μm, which makes them able to pass through the stratum corneum (thickness between 10 and 30 μm).
Furthermore, they are responsible of forming pores in the skin, which are large enough to allow macromolecules to pass through, simply and painlessly.
Despite the advantages of this notable approach, microneedles have an obstacle when it comes to the delivery of substances: the elasticity of the skin. This parameter may hinder the penetration of microneedles in the stratum corneum, since the skin, can deform with the pressure exerted by the needle, without breaking its barrier. Consequently, pore formation and substance permeation are compromised.
There are some studies suggesting that different microneedles allow successful permeation of peptides into the skin. An in vitro study was performed by Zhang et al., to investigate the efficacy of solid microneedle arrays (consisting of 121 needles, attached to an applicator) in delivering hydrophilic peptides, namely acetyl hexapeptide-3, into pig ear skin. The results of the study exhibited not only that this physical system was effective in forming pores, but also in the delivery of the peptides through the skin, since the passive flow of acetyl hexapeptide 3 through the skin, when microneedles were applied was 0.44 ± 0.12 μmoL/cm/h, which was much higher than the passive flow of this peptide in untreated skin (0.014 ± 0.002 μmoL/cm/h).
Source: Journal of Drug Delivery Science and Technology - Anti-aging peptides for advanced skincare: Focus on nanodelivery systems

Microplastics are tiny pieces of plastic that measures less than five millimeters in size. Some microplastics are formed by breaking away from larger plastics that have fragmented over time. Others are made intentionally small, otherwise known as microbeads. These are also used in cosmetics products such as face scrubs, as well as detergents, paints, medicines, nappies and pesticides. The use of microbeads in cosmetics is banned in the EU.

Minimum order quantity is a requirement from some manufacturers for the minimum number of orders to be purchased at one time. This is required in order for manufacturers to remain profitable, as often they work on smaller margins and require large order volumes to generate enough revenue to maintain their business.
The MOQ is typically between 5 to 10,000 pieces for custom cosmetic packaging, and for cosmetic RAW materials, it varies between 1 to 50 kgs, depending on the supplier of the ingredient. Contract manufacturers also have an MOQ, which typically ranges from 2,000 to 10,000 pieces, depending on the manufacturer.

The term ‘mineral oil’ refers to very highly refined liquid hydrocarbons derived from petroleum distillates, which are used in medicine, pharmaceuticals, cosmetics, food packaging, food contact applications and food itself. Other terms often used interchangeably with mineral oil, include ‘liquid petrolatum’, ‘liquid paraffin’, ‘paraffin oil’, ‘medicinal oil’, ‘white oil’, ‘white mineral oil’, ‘food grade oil’, ‘food grade white oil’ and ‘technical white oil’. Mineral oils (medium and low viscosity) are manufactured from crude mineral oils in various refining steps, such as distillation, extraction and crystallization, and are subsequently purified by acid treatment (oleum method) and/or hydrotreatment (catalytic hydrogenation). Mineral oils (medium and low viscosity) are mixtures of highly refined paraffinic and naphthenic liquid hydrocarbons with boiling points greater than 200°C. They are lightweight, inexpensive, odourless and tasteless. Mineral oils are a common ingredient in baby lotions, cold creams, ointments and cosmetics. Examples are their use to prevent brittleness and breaking of eyelashes, in cold cream, and to remove make-up and temporary tattoos. A common concern regarding mineral oil is the presence in many lists of comedogenic (i.e. clogs skin pores) substances that were developed many years ago and are frequently quoted in the dermatological literature. However, more recently, highly refined and purified oils commonly used in cosmetics and skin care products are non-comedogenic.
Source: Cosmetic Formulation Principles and Practice - Heather A.E. Benson, Michael S. Roberts, Vânia Rodrigues Leite-Silva, Kenneth A. Walters
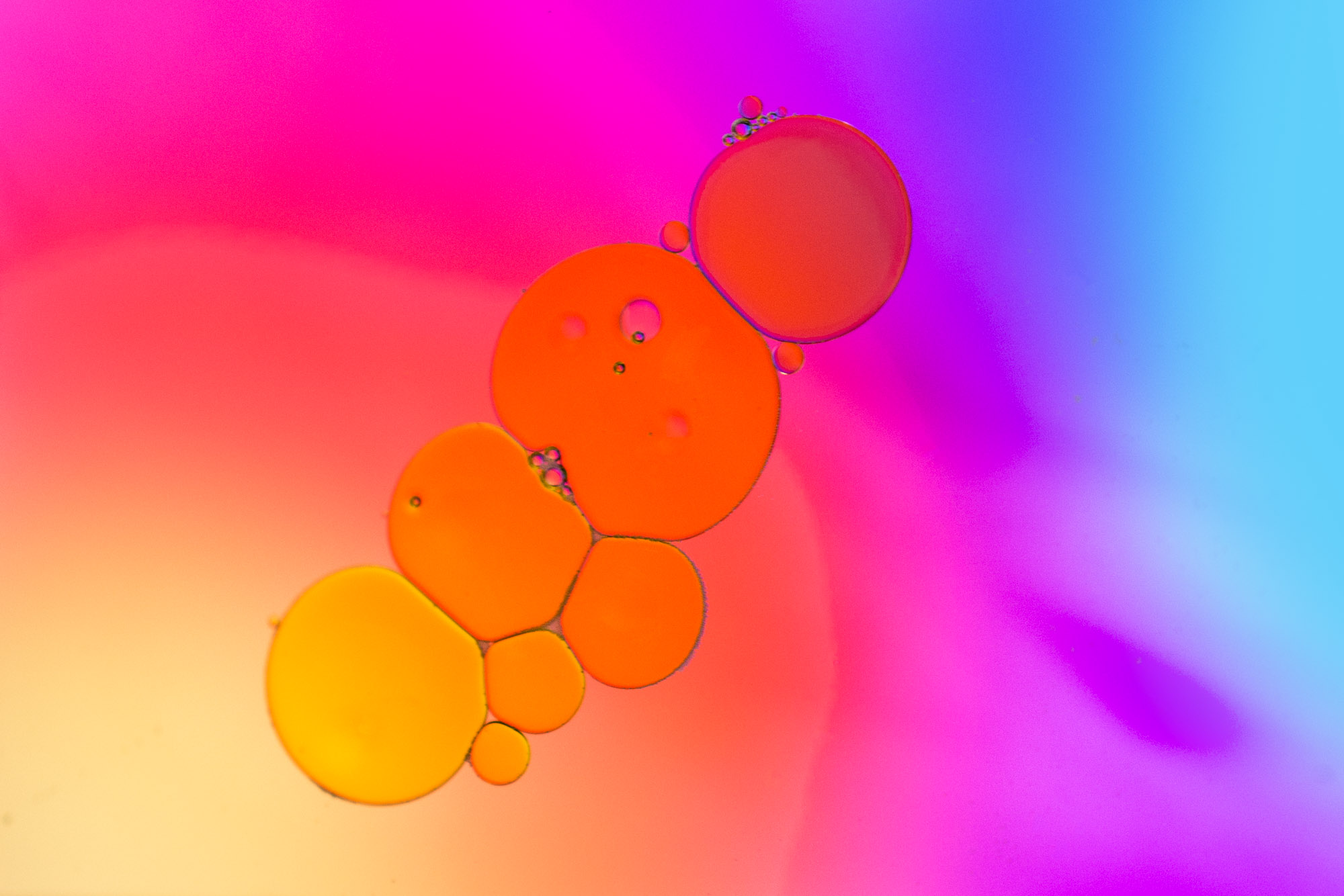
Microemulsion is defined as a system of water, oil, and amphiphile, which is a single optically isotropic and thermodynamically stable liquid solution. ‘‘This definition should be widened, however, to include metastable states, spontaneous emulsions of long-lived kinetic stability.’’ The term microemulsion may be a misnomer, because microemulsions consist of large or ‘‘swollen’’ micelles containing the internal phase, much like that found in a solubilized solution.
Microemulsions contain oil droplets in a water phase or water droplets in oil with diameters of about 10 to 200 nm. Therefore they appear as isotropic, optically clear liquid or gel-like systems. Unlike micellar solubilized systems, microemulsions may not be thermodynamically stable; nevertheless, they are more stable than ordinary emulsions. They are a type of ternary system composed from water, lipid, and surfactant mixture in a distinct ratio
Microemulsions may be used to incorporate or dissolve active substances and have been found to improve skin penetration and permeation.
The disadvantage of microemulsions is their rather high concentration of surfactants, which is a risk for increased skin irritation and sensitization. Nevertheless, modern microemulsion formulation is based on alkyl polyglycosides which are regarded to be milder than conventional nonionic surfactants with polyoxyethylene chains.
Hydrogels are hydrophilic, consisting mainly (85–95%) of water or an aqueous-alcoholic mixture and the gelling agent.
Source: Handbook of Cosmetic Science and Technology - André O. Barel, Marc Paye, Howard I. Maibach

Peppermint. Country: South Africa/Malawi. Part used: fresh leaves. Traditional use: Typical minty fragrance with mentholic undertones. It has a clean, clearing, penetrating odor. Invigorating; ideal travel companion, calms the stomach. Used to bathe tired and sweaty feet. A good insect repellent. Has a cooling effect on the body. Stimulating, used for headaches and nausea, very cooling. Breath freshener.
Source: Handbook Of Natural Ingredients - Anthony C. Dweck

A spherical conglomeration of surface active molecules formed in solution. The polar groups on the molecules align toward the water phase and the nonpolar groups tend to point toward the oil or nonpolar phase. This phenomenon allows groups of molecules to form spherical cells. Micelle formation is the basis for most emulsification. Liposomes are a form of micelles.
The diameter of a Micelle particle ranges from 5 to 100 nm.
The size and shape of micelles can vary depending on the type of surfactant and the conditions in the solution.
Micelles can be either spherical or rod-shaped.
Micelles can be used to solubilize a variety of different substances, including oils, fats, and drugs.
Micelles can also be used to stabilize emulsions and foams.

Microcrystalline wax is a solid obtained by extracting the oil from petrolatum. It is a complex mixture composed mainly of C31–C70 isoparaffins. It has a microcrystalline structure, high adhesive power, good extensibility, is not susceptible to low temperatures and has a high melting point (60–85°C). When mixed with other waxes, it suppresses crystal formation making it useful in lipsticks and creams.
Source: Cosmetic Formulation Principles and Practice - Heather A.E. Benson, Michael S. Roberts, Vânia Rodrigues Leite-Silva, Kenneth A. Walters

Melasma manifests as increased pigmentation in the face, typically symmetric patches or macules are found on either side, with a tendency to occur on the sunexposed
areas of the face (cheeks, upper lips). This disorder is much more common in women than men (a ratio of 9:1) and in persons with high-grade phototype.
Major etiologic factors include genetic influences, female sex hormones, and exposure to UV radiation. Histopathology findings indicate hyperpigmentation in all epidermal layers caused by an increase in melanin and also the number of active melanocytes. A flattening of the epidermal rete ridges has been reported, suggesting that keratinocytic proliferation may not be involved in melasma. Pathogenesis is poorly understood. A recent study indicated that the high expression of α-MSH in keratinocytes of the melasmic lesions was a major factor. Treatment options are prevention using good broad-spectrum sunscreen, retinoids, α-hydroxy acids, and hydroquinone.
Source: Dermatologic, Cosmeceutic and Cosmetic development - Kenneth A. Walters, Michael S. Roberts

Letterpress printing in commercial printing, process by which many copies of an image are produced by repeated direct impression of an inked, raised surface against sheets or a continuous roll of paper. Letterpress is the oldest of the traditional printing techniques and remained the only important one from the time of Gutenberg, about 1450, until the development of lithography late in the 18th century and, especially, offset lithography early in the 20th.
Originally the ink-bearing surface for printing a page of text was assembled from individual types by a typesetter or compositor, letter by letter and line by line. The first keyboard-actuated typesetting machines, the Linotype and the Monotype (qq.v.), were introduced in the 1890s. If only a small number of copies is to be made, printing can be done directly from the hand- or machine-set blocks of type assembled in forms, but for long press runs, duplicates—stereotypes or electrotyping (qq.v.)—are made to prevent wear and damage of the expensive types.
Letterpress was originally carried out on platen presses, in which the paper is pressed against the flat, inked form by a flat platen; later, the platen was replaced by a roller in the flat-bed cylinder press; still later, the printing form was wrapped around one cylinder and the paper was passed between this cylinder and a second, creating a rotary press (see printing).
Letterpress can produce work of high quality at high speed, but it requires much time to adjust the press for varying thicknesses of type, engravings, and plates. Because of the time needed to make letterpress plates and to prepare the press, many newspapers have changed to offset printing. To combat this trend, letterpress printers have developed printing plates made from a photosensitive plastic sheet that can be mounted on metal.
Source: Britannica.com

Mango is a tree that grows up to 40 m in height. It originates from the regions of Bangladesh, Myanmar, India and Indonesia. It is one of the most exploited tropical trees, both in its native regions and in Africa, Australia and South America, where the tree is grown commercially. Botanical characteristics: bark greyish brown or black; leaves smooth, initially red, but become dark green during growth, lanceolate; inflorescence paniculate, flowers small, greenish white or pink, in groups of 500 to 6,000, petals 5, stamens 5, sepals 5, pubescent; fruits botanically termed drupes, oblong, 8 to 12 cm long, exocarp smooth, greenish yellow or greenish red, mesocarp fleshy, juicy, yellow, endocarp hard, with 1 large seed.
Mango seeds contain 9 to 13% butter. Its triglyceride composition is characterised by the balanced proportion of predominant stearic and oleic acids. Another important feature is its content of phytosterols, which may be as high as 7%. The melting point is approximately 35°C. Mango butter is stable against oxidation. It is of yellow or light-brown colour and has a typical sweetish-oily odour.
Given its fatty acid composition, mango butter is most similar to shea butter and is considered its best substitute. However, the consistency of mango butter is slightly more solid and it contains less unsaponifiable matter.
Mango seeds contain up to 50% water and must therefore be quickly dried after harvesting to reduce the water content to approximately 10%. The harvesting period for mango fruit in the countries of southwest Asia, which are the leading producers of mango butter, coincides with the monsoon season. Such demanding climatic conditions make the processing of mango fruit very difficult. Wet seeds are suitable for the extensive development of microorganisms, especially Aspergillus niger. Fungus lipases degrade triglyceride molecules into free fatty acids. In unrefined mango butter produced from fresh seeds, the content of free fatty acids was shown to increase from 2 to 7% in 20 days, and to 46% in 120 days.
Mechanism of action and use
Mango butter is used as an emollient ingredient in a variety of cosmetic products, e.g. skin and hair cleansing cosmetics, skin and hair care cosmetics, lip care cosmetics and decorative cosmetics. It is also very popular in products for massage. Due to its high content of phytosterols, it may contribute to antioxidative activity and restore the impaired function of the lipid barrier. In vivo laboratory studies with an emulsion containing 25% mango butter have shown accelerated wound healing. Similar effects have been observed with the same emulsion used in volunteers. Scientific literature describes some very rare cases of contact dermatitis caused by the dermal use of mango butter.
Source: Modern Cosmetics - Dr. Damjan Janeš and Dr. Nina Kočevar Glavač

Lecithin is a natural mixture of polar and neutral lipids; the word lecithin is also used as the trivial name of a particular phospholipid: phosphatidylcholine. Main vegetable
sources of lecithin used in personal-care products are soybean and maize, egg yolk is practically the only animal source of lecithin used in cosmetics and toiletries. The percentage of polar lipids and their fatty acid pattern are characteristic of the lecithin source.
Bare lecithin, a secondary product of Soya oil extraction, typically contains 60 to 70% polar lipids (mainly phospholipids, namely phosphatidylcholine, and glycolipids) and a remaining 25 to 35% Soy oil. This raw lecithin is further fractionated, purified, and chemically modified to allow easier processing and formulation in toiletry products. Emollient, refattening, and moisturizing properties of lecithin are guided by its content in phospholipids.
Lecithin softens, nourishes, and refattens the skin; it provides a nongreasy, long-lasting skin feel and improves foam feel and quality (creaminess, slipperiness, richness).
Ready-to-use mixtures of phospholipids in surfactant solutions, free of residual Soya oil, are commercially available for an easy incorporation in liquids or bars; some of these compounds allow formulation of clear products.
Source: Handbook of Cosmetic Science and Technology - André O. Barel, Marc Paye, Howard I. Maibach

Lanolin is extracted from sheep wool grease; it is a complex mixture of esters of high molecular weight lanolin alcohols (aliphatic alcohols, sterols, and trimethyl sterols) and of lanolin acids; free lanolin alcohols, acids, and lanolin hydrocarbons are minors. Lanolin alcohols and lanolin oil are recommended as superfatting agents in soaps.
Ethoxylation of the hydroxyl groups of lanolin or of its derivatives leads to hydrophilic, water-soluble lanolin compounds, offering a broad range of useful emollients to the formulator. Some moderately to highly ethoxylated derivatives, recommended for their good emolliency and moisturization properties, are processable in liquid skin cleansers with limited impact on foam profile; as an example, the 75 mol ethoxylated lanolin does not depress foam and is recommended as skin conditioner in soaps, liquid body-cleansing products, and bubble baths. Medium ethoxylates lanolin alcohols have limited impact on foam performances of body cleansing liquids; lower ethoxylates can be formulated in bars.
Propoxylated lanolin alcohols are lipophilic emollients used in soap bars and in other cleansers based on synthetic surfactants. Alkoxylated lanolin derivatives are obtained by reaction with mixtures of propylene and ethylene oxides in various ratios; they are more soluble than ethoxylated lanolin. They serve as refattening and foam stabilizing agents. Esterification of lanolin fatty acid with isopropyl alcohol provides a range of esters of various molecular weights. Medium molecular weight esters are used as superfatting agents in soaps.
Source: Handbook of Cosmetic Science and Technology - André O. Barel, Marc Paye, Howard I. Maibach

Lavandula Angustifolia Water is an aqueous solution of the steam distillate obtained from the Lavender, Lavandula angustifolia, Labiatae. is distilled from the flower buds of Lavandula angustifolia. It has the fresh, clean scent of lavender has been popular for centuries. The plant is often used as a gentle tonic for the nervous system and is said to soothe headaches and other aches and pains. The soothing and antiseptic qualities make lavender useful in all skin care. This material from the condensate of the distillation of the essential oil contains water soluble constituents as well as a small amount the essential oil and has a strong Lavender scent. It may be used alone as a facial tonic/toner or in blends to soothe irritated or burned skin. No adverse effects are expected or have been reported from the topical use of this material..
Source: Dweck, Anthony. Handbook of Natural Ingredients (Dweck Books 4) . Dweck Data.

Lactic acid is found in nature in milk, it is also found in the skin. The role of lactic acid is to improve the integrity of the acid mantle of the skin and it acts as part of the skin’s natural moisturising function. It not only acts as an astringent, but also helps to moderate the pH of the skin to keep it slightly the acidic (the ideal value). Lactic acid is used in skin softening preparations and has been used at higher levels for the treatment of thickened skin conditions (e.g. calluses, warts, etc). The SCCNFP adopted a position paper (SCCNFP/ 0370/ 00) (1) on the safety of AHA based on studies on short term phototoxicity (sensitivity of human skin to UV-induced damage: sunburn cells and pyrimidine dimers production) and skin irritation. The SCCNFP considered that there was a need for more information in order to provide a full scientific assessment of the safety of AHA. However, on the precautionary principle, the SCCNFP suggested that: lactic acid up to a maximum level of 2.5 % and a pH ≥ 5.0. [CAS: 50-21-5; EINECS: 200-018-0]. Function: Buffering/ humectant/ skin conditioning. The actual or estimated LD50 value: 3,543 mg/kg body weight. AICS status (NICNAS Australia): AICS Compliant. Oral LD50 value (rat): 3,543 mg/kg. Dermal LD50 value (rabbit): 2,000 mg/kg. Lactic Acid. CIR: Concentration or other limitation on use for safe with qualifications conclusion: </=10%, at final formulation pH>/=3.5, when formulated to avoid increasing sun sensitivity or when directions for use include the daily use of sun protection;</=30%, at final formulation pH>/=3.0, in products designed for brief, discontin-uous use followed by thorough rinsing from the skin, when applied by trained professionals, and when application is accompanied by directions for the daily use of sun protection.
Source: Dweck, Anthony. Handbook of Natural Ingredients (Dweck Books 4) . Dweck Data.

Lactobacillus Ferment is the product obtained from the fermentation of Lactobacillus Lactobacillus fermentum is a Gram-positive species of bacterium in the genus Lactobacillus. It is associated with active dental caries lesions. It is also commonly found in fermenting animal and plant material. It has been found in sourdough. A few strains are considered probiotic or "friendly" bacteria in animals and at least one strain has been applied to treat urogenital infections in women. A patent was issued that claimed The present invention concerns methods and compositions for prophylactic or therapeutic treatment of skin disorders by administering therapeutically or prophylactically effective amounts of a probiotic and is also concerned with the treatment of symptoms of skin disorders. Lactobacillus is used for skin disorders such as fever blisters, canker sores, eczema (allergic dermatitis); and acne. It is also used for high cholesterol, lactose intolerance, Lyme disease, hives, and to boost the immune system. Women sometimes use lactobacillus suppositories to treat vaginal infections and urinary tract infections (UTIs). In light of the evidence there are few concerns for the topical application of this material.
Source: Dweck, Anthony. Handbook of Cosmetic Ingredients: - their use, safety and toxicology (Dweck Books 5)
A cosmetic leak test is a test that is conducted to ensure that the packaging of a cosmetic product is airtight and does not leak. The test is done by pressurizing the package and then looking for a drop in pressure over time.
The purpose of a cosmetic leak test is to ensure that the product is safe to use and that it will not spoil or become contaminated during its shelf life. The test also helps to prevent the product from drying out or evaporating.
There are a few different methods that can be used to conduct a cosmetic leak test. One common method is to use a differential pressure decay leak tester. This type of tester uses a pump to pressurize the package and then measures the rate at which the pressure drops over time. A leak will cause the pressure to drop more quickly.
The cosmetic leak test is an important part of the quality control of cosmetic products. It helps to ensure that the products are safe and effective for consumers to use.

Kojic acid, the chemical name 5-hydroxy-2-hydroxymethyl-4-pyrone, is used in cosmetic products as a skin whitening or depigmenting agent. Kojic acid is a chelating agent produced by several species of fungi, especially Aspergillus oryzae, which has the Japanese common name of koji. It is a by-product in the fermentation process of malting rice, when producing sake (Japanese rice wine). It is used in food and cosmetics to help preserve against color changes. Kojic acid also has antibacterial and antifungal properties. Kojic acid markedly inactivated isolated tyrosinase by chelation. In cultured human melanocytes, tyrosinase activity per well was slightly reduced at the concentration range between 0.1 mM and 0.5 mM but was rapidly dose-dependently reduced at higher concentration. The inhibitory effect of kojic acid on tyrosinase activity in the cell culture system is smaller than that of arbutin at concentrations that do not affect cell viability, even though marked inactivation was observed in isolated tyrosinase. There are conflicting reports on the effectiveness in kojic acid. [Maeda and Fukuda]. Kojic acid may take two to three months to show efficacy and thus may seem rather slow to be effective. However, kojic acid does not have any side effects during the 10- to 20-month period during normal application on the skin. Kojic dipalmitate is mentioned in the Inventory of Cosmetic Ingredients, but derivative esters of Kojic acids are also used. The substance is listed as an emollient, whereas Kojic acid itself is listed as an antioxidant. Results of the range finding test indicated that the LD50 was in the range of 4000 to 16000 mg/kg bw. In the main experiment lethargy, piloerection, abnormal body carriage, ataxia and depressed respiration rate were observed shortly after dosing. These signs were accompanied by gasping amongst mice treated at 6400 mg/kg bw. Bodyweight increases of rats treated at 16000 mg/kg bw were slightly depressed during the first week. Recovery of survivors was apparently complete within four days of dosing. Autopsy revealed congestion of the lungs and pallor of the liver, kidneys and spleen in animals died after treatment. The LD50 and its 95% confidence limits were calculated to be 5100 (3900 – 6700) mg/kg bw. No erythema or oedema occurred in the test performed. Kojic acid was not considered to be an irritant to rabbit skin. In the preliminary test and in the first experiment, 3% Kojic acid aqueous solution caused no eye disturbances. In the second experiment mild transient hyperemia was observed in 2 of 4 animals. No other inflammatory changes or corneal disturbances were observed. Eye irritability was reported to be very weak. In the supplementary test no specific response was observed for up to 72 hours. Two out of 20 animals showed a positive reaction, indicating a sensitising potential of the substance. Kojic acid is sensitising in humans. Based on the information provided, margins of safety of respectively 35 (face and hands), 58 (hands) and 88 (face) have been calculated suggesting that the use of Kojic acid at a maximum concentration of 1.0% in skin care formulations poses a risk to the health of the consumer. In addition, other parts of the skin might be exposed to Kojic acid. Kojic acid has the potential to induce skin sensitisation. Relevant data on kinetics of Kojic acid after dermal application may be submitted to refine the MOS approach..
Source: Dweck, Anthony. Handbook of Natural Ingredients (Dweck Books 4) . Dweck Data.

Inositol is a naturally occurring nutrient that is usually classified as a carbocyclic polyol. The most common form of inositol is sometimes referred to as myo-inositol. In the human body, inositol plays a major role in preventing the collection of fats in the liver, as well as promoting healthy hair growth. The presence of the nutrient also aids in efficient processing of nutrients into the conversion of energy, which in turn helps the body to maintain a healthy metabolism. [CAS: 87-89-8; EINECS: 201-781-2]. Function: Antistatic/ hair conditioning/ humectant. The actual or estimated LD50 value: 10,000 mg/kg body weight. AICS status (NICNAS Australia): AICS Compliant. [Panthenyl Ethyl Ether, Milk Protein, Lactose, Inositol, Acetyl Cysteine, Acetyl Methionine, Sodium Citrate, Citric Acid]. Follicusan™ contains biologically active signaling proteins, ethyl panthenol (provitamin B5), inositol, as well as acetyl cysteine and acetyl methionine, in a water-alcohol medium. The product vitalizes the cells of the scalp, including the hair follicles, and thus counteracts premature, accelerated hair loss (alopecia). Follicusan™ is a whitish, opaque liquid and dissolves in water and (used at the recommended maximum concentration of 5 %) dissolves in water-alcohol mixtures with a maximum alcohol content of 50 % w/w. Within the pH range 3.5 to 5.5 the proteins in Follicusan™ may precipitate, therefore when formulating with Follicusan™ this pH range should be avoided.
Source: Dweck, Anthony. Handbook of Natural Ingredients (Dweck Books 4) . Dweck Data.

What is injection moulding?
Injection moulding is the process of manufacturing highly accurate plastic components such as closures, overcaps, and reducing plugs. Plastic resin is fed into a heated barrel and screw which is then injected at high pressure into a water-cooled mould which may consist of various moving parts.
Due to the high injection pressures, this cavity will be held together by the use of a clamping unit. While the plastic is held inside the mould, the plastic will freeze off to the desired shape. After the plastic has cooled sufficiently, the mould and clamping unit will open and allow the finished component to be ejected so that the process can be repeated.
Which materials can be injection moulded?
Polypropylene (PP) is the most common resins used in injection moulding and can be divided into three sub categories:
- Homo polymer: For general use of moulding closures
- Co Polymer (Random): Gives excellent clarity when used for overcaps and when closures are used with active hinges as with 'flip top' closures
- Co Polymer (Block): These components will generally have poorer clarity and are therefore generally coloured components but offer greater impact resistance
Generally PP is advantageous when being used in conjuction with a dissimilar bottle materials such as HDPE, PVC or PETG as it will improve any binding issues between the two components.

Injection Stretch Blow Moulding (ISB) is a process that manufacturers high quality containers in PET to produce a clarity similar to glass.
ISB can also produce materials that are less clear such as polypropylene (PP).
Production process
Production begins with the injection process, where molten polymer flows into an unjection cavity to produce a preform, a hollow test tube shaped plastic object that has a neck and a thread on the open end.
When the preform is conditioned to the correct temperature, it is ready for stretching and blowing to the desired shape using a mould. Once in the mould, a rod is introduced to stretch the preform using two levels of air pressure, the preform is then blown circumferentially. After a set cooling time, the moulds open and the preformed bottle is removed. The process is carried out concurrently using a revolving carousel of moulds.
ISB is an environmental alternative to glass
Because plastics are soft and boast lower melting points, PET bottles require less energy to manufacture than glass. Easier to recycle, PET can also be made in PCR in levels up to 100%.
It is also possible to make PET products using a range of biopolymers, including sugarcane based PET. With no sign of difference in both appearance or performance, out PET biopolymer can be recycled in the same stream as conventional PET and PCR PET, for a truly sustainable solution.
What are the advantages of this process?
Offering dimensionally accurate bottle shapes, when molded, PET (Polyethylene Terephthalate) provides an excellent alternative to glass for a number of reasons
These include:
- Clarity similar to glass
- Lighter in weight
- More cost effective to transport products
- Higher breakage resistance to avoid risk and injury
- More durable than glass
- Excellent barrier characteristics against carbon dioxide and oxigen
- Requires less energy to produce
- Can be easily recycled
- PET can be decorated with silk screen printing and hot foil blocking
What is INCI?
INCI names (International Nomenclature Cosmetic Ingredient) are systematic names internationally recognized to identify cosmetic ingredients. They are developed by the International Nomenclature Committee (INC) and published by the Personal Care Products Council (PCPC) in the International Cosmetic Ingredient Dictionary and Handbook, available electronically as wINCI.
Oversight for the INCI program is provided by PCPC as part of its mission to support the identification of the composition of personal care products, and publication of this information in a worldwide science-based Dictionary. PCPC is committed to ensuring that the Dictionary provides the world community with accurate, transparent, and harmonized nomenclature. By working closely with its international sister trade associations, and with other organizations around the world, PCPC strives to develop INCI names that accommodate differing labeling approaches described in national laws and regulations.
Why INCI?
There are many benefits to a uniform system of labeling names for cosmetic ingredients. Dermatologists and others in the medical community are ensured an orderly dissemination of scientific information, which helps to identify agents responsible for adverse reactions. Scientists are ensured that information from scientific and other technical publications will be referenced by a uniform name; and that multiple names for the same material will not lead to confusion, misidentification, or the loss of essential information. It also enables the cosmetic industry to track the safety and the regulatory status of ingredients efficiently on a global basis, enhancing its ability to market safe products in compliance with various national regulations. And finally, transparency is provided to consumers as ingredients are identified by a single labeling name regardless of the national origin of the product.
What INCI is not?
The designation of an INCI name for a cosmetic ingredient is an essential part of ingredient identification; however, just because an ingredient has an INCI name does not mean that the ingredient has been approved for cosmetics. The assignment of an INCI name to an ingredient also does not imply that the ingredient is safe, or that its use in a cosmetic product complies with the laws and regulations of the United States or other global regions. The safety and fitness of use for an ingredient, along with regulatory considerations, is carefully evaluated by the manufacturer as part of the development process before the product is marketed.
Source: https://www.personalcarecouncil.org/

Hydrogen Peroxide is a clear, colorless liquid. In cosmetics and personal care products, Hydrogen Peroxide can be found in a wide variety of hair care products such as hair dyes, hair bleaches, conditioners, shampoos and rinses, hair bleaches and shampoos. It is also used in tooth whitening products. Hydrogen Peroxide is also sold as an antiseptic at concentrations of 2.5-3%. The Food and Drug Administration (FDA) includes Hydrogen Peroxide in its list of substances affirmed Generally Recognized As Safe (GRAS) for use in food. Hydrogen peroxide is used as an antimicrobial agent, an oxidizing and reducing agent, and bleaching agent in foods such as milk and cheese products, wine, vinegar, starch and instant tea. The FDA also allows Hydrogen Peroxide to be used in Over-the-Counter (OTC) first aid antiseptics. Hydrogen Peroxide is added to cosmetics and personal care products as an antimicrobial agent and as an oxidizing agent. The function of antimicrobial agents is to kill or inhibit the growth or reproduction of microorganisms. In cosmetics and personal care products, oxidizing agents are used to form dyestuffs during oxidative hair dyeing, and to oxygenate stains on the teeth to further whiten the teeth.
Source: Dweck, Anthony. Handbook of Cosmetic Ingredients: - their use, safety and toxicology (Dweck Books 5)

Hydrolyzed Wheat Starch is a hydrolysate of wheat starch derived by acid, enzyme or other method of hydrolysis. Naturally derived, hydrolyzed wheat starch contains wheat oligosaccharides and constitutes a unique hydrating complex offering a combination of moisture-balancing and film-forming properties that work synergistically to give hair better body control, and skin, a smoother softer feel. There are no adverse effects reported or expected from this well-established hair and skin softener.
Source: Dweck, Anthony. Handbook of Cosmetic Ingredients: - their use, safety and toxicology (Dweck Books 5)



